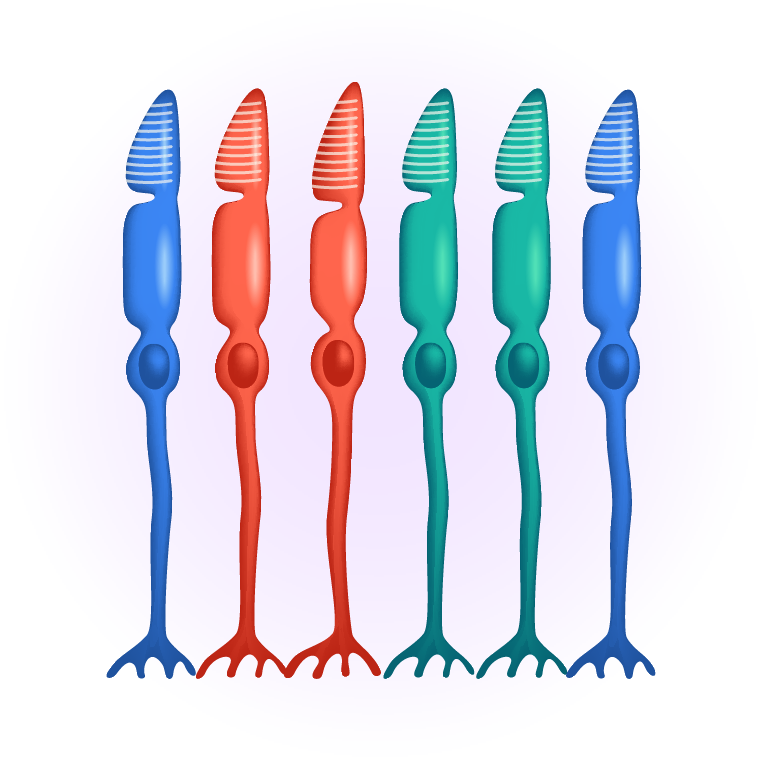
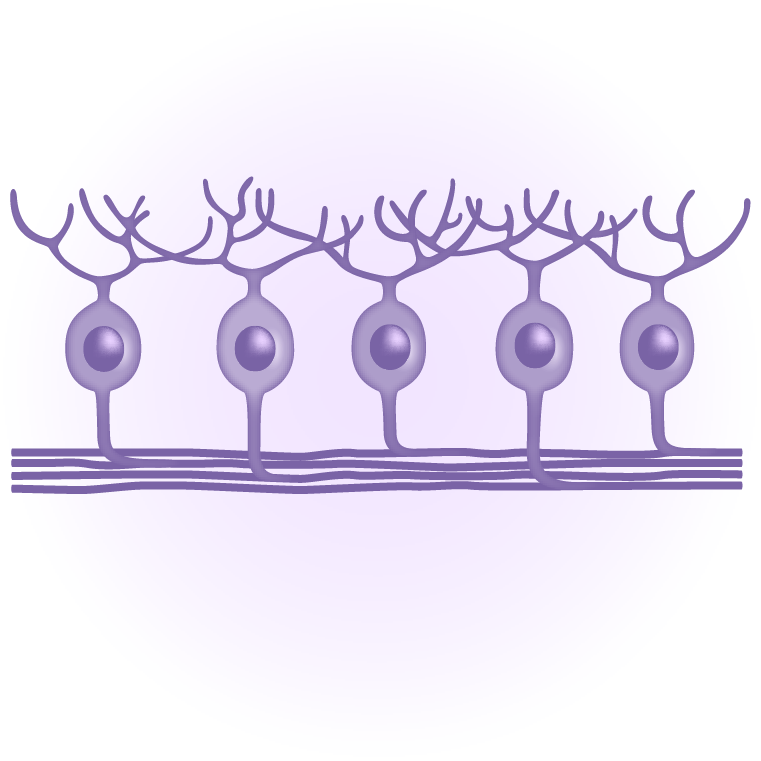
Regenerative cell therapy can restore the machinery of vision
Vision loss is a growing cause of disability, with the global prevalence of age-related macular degeneration expected to reach 288 million individuals, globally, by 2040. In virtually every ocular disease, vision deteriorates due to the damage or loss of cells in the eye. Since in many cases the cells of the eye such as photoreceptors, retinal pigment epithelium and retinal ganglion cells are unable to regrow on their own, replacement through engineered cell-based therapeutics is the only treatment modality with the potential to dramatically improve vision. This is particularly true in later-stage disease where a significant proportion of tissue/cells are already lost, leaving no anatomy to benefit from conventional treatments, including gene therapies.
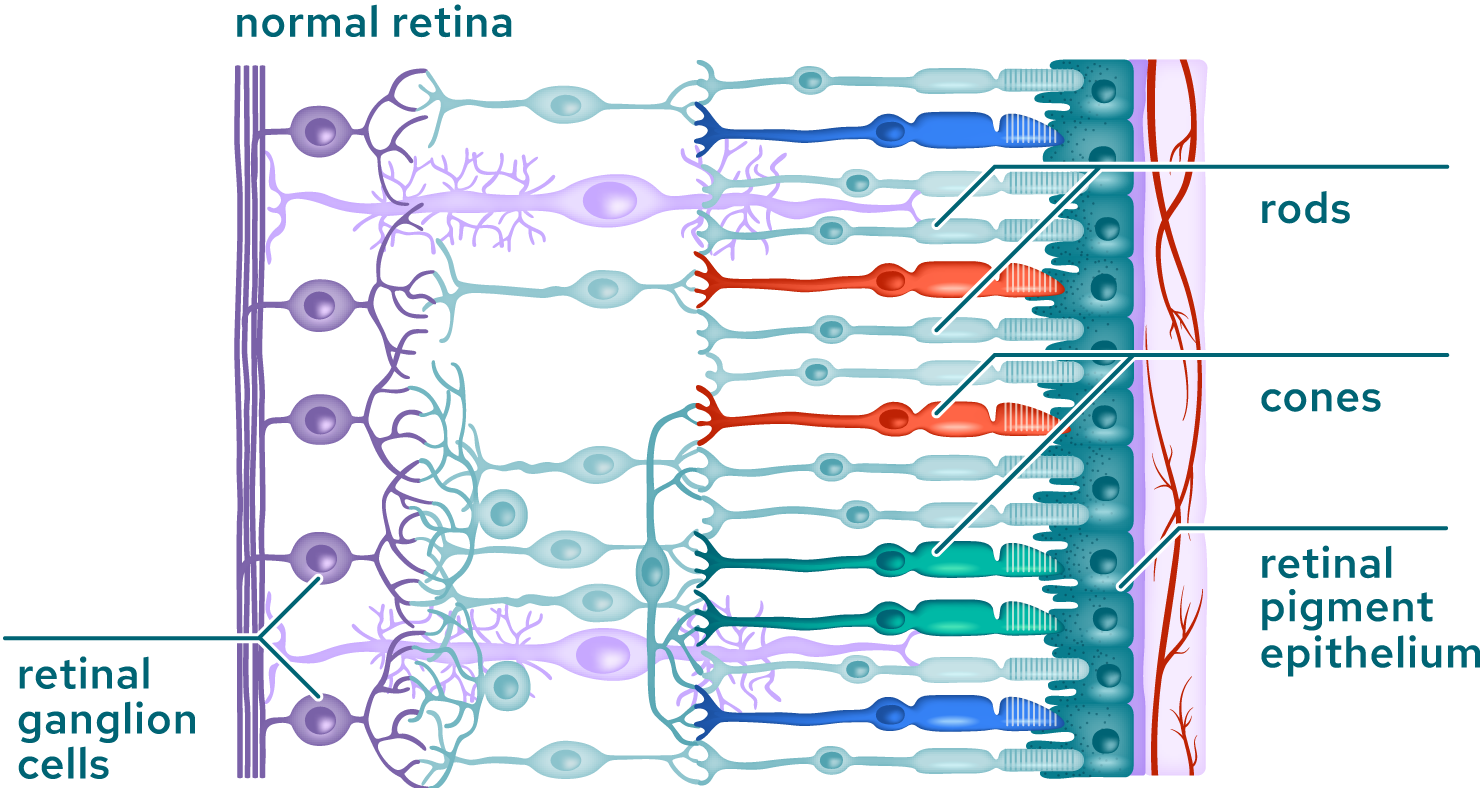
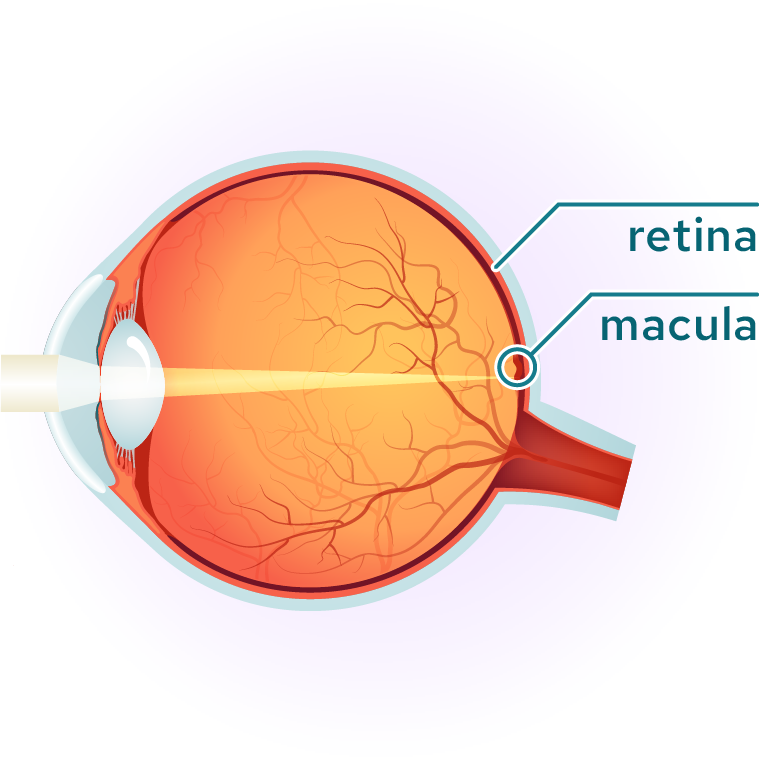
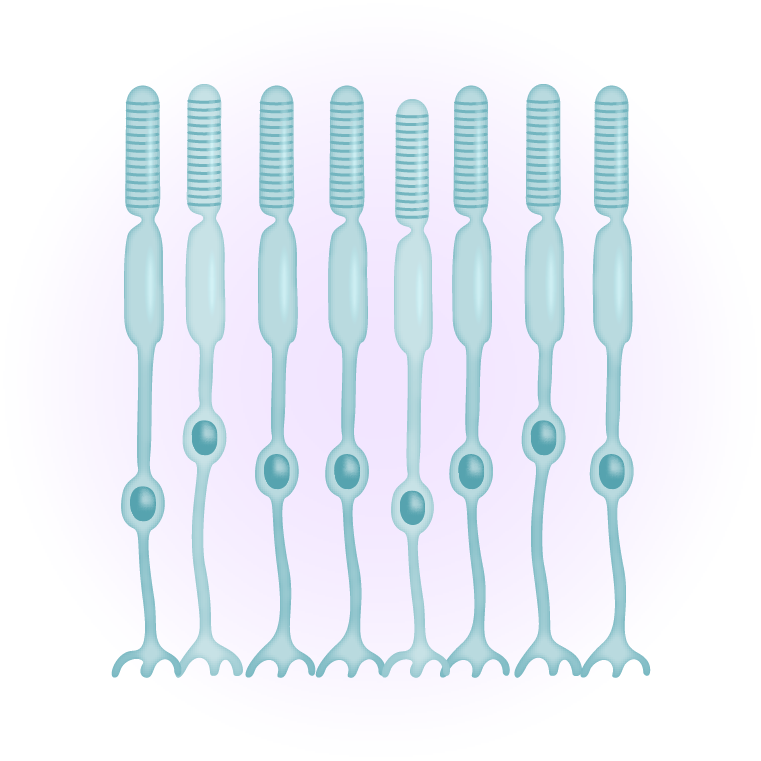
The macula is the area of the retina where the photoreceptors, mostly cones, are most concentrated. It is a small area of the retina, about five millimeters across, where all high-resolution vision is located and is responsible for daylight and color vision. An intact macula is essential for a wide range of everyday tasks such as reading and driving. Outside the macula, in the peripheral retina, photoreceptors are less densely packed and dominated by rod photoreceptors, which are responsible for peripheral and low-light vision.
Our Processes
The eye is an ideal location to study the impact of regenerating function through replacing lost or dysfunctional cells due to its small size, immune privilege, low dosing requirements and other features. Tenpoint uses both ex vivo and in vivo approaches to replace damaged or missing cells.
Ex vivo
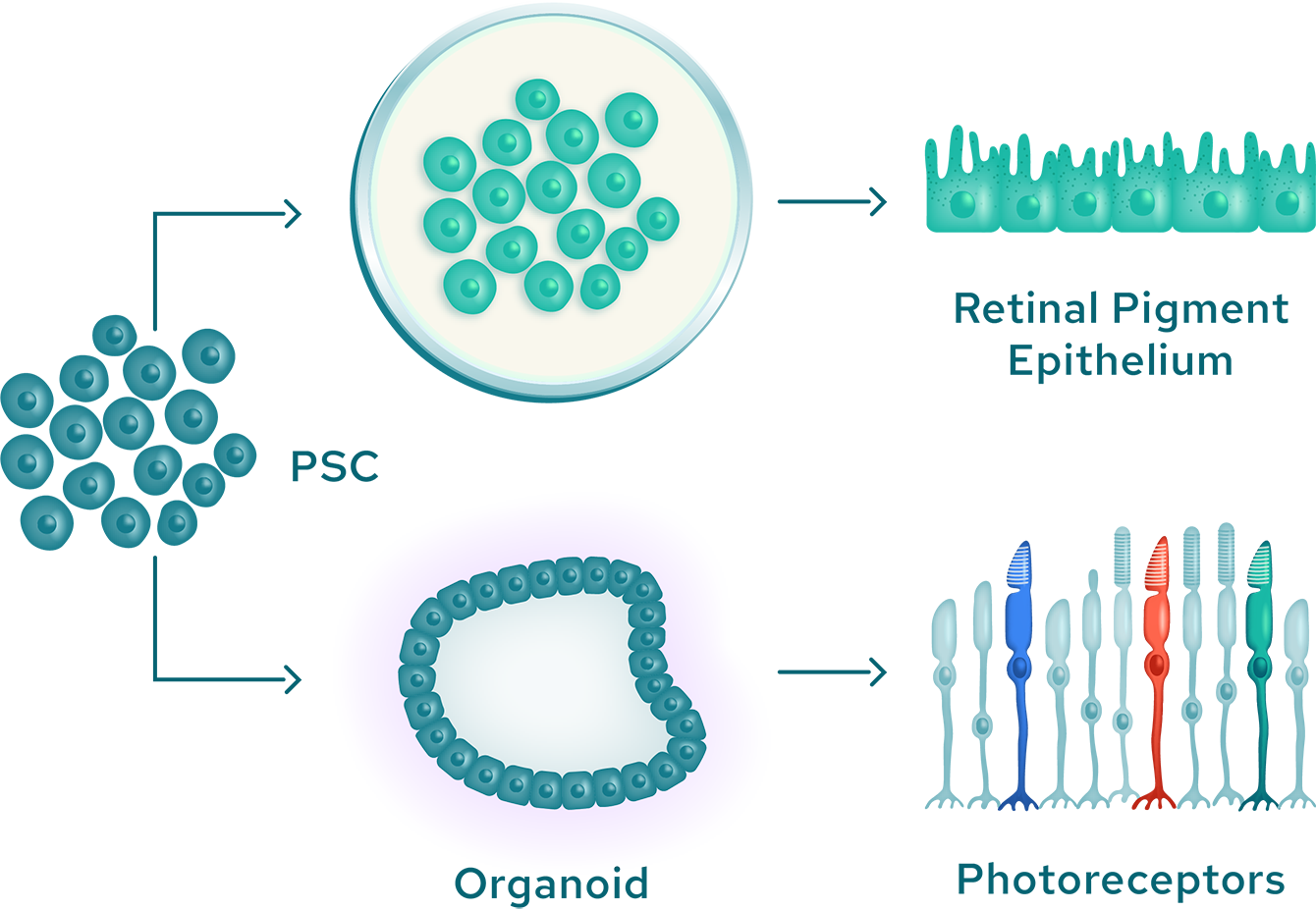
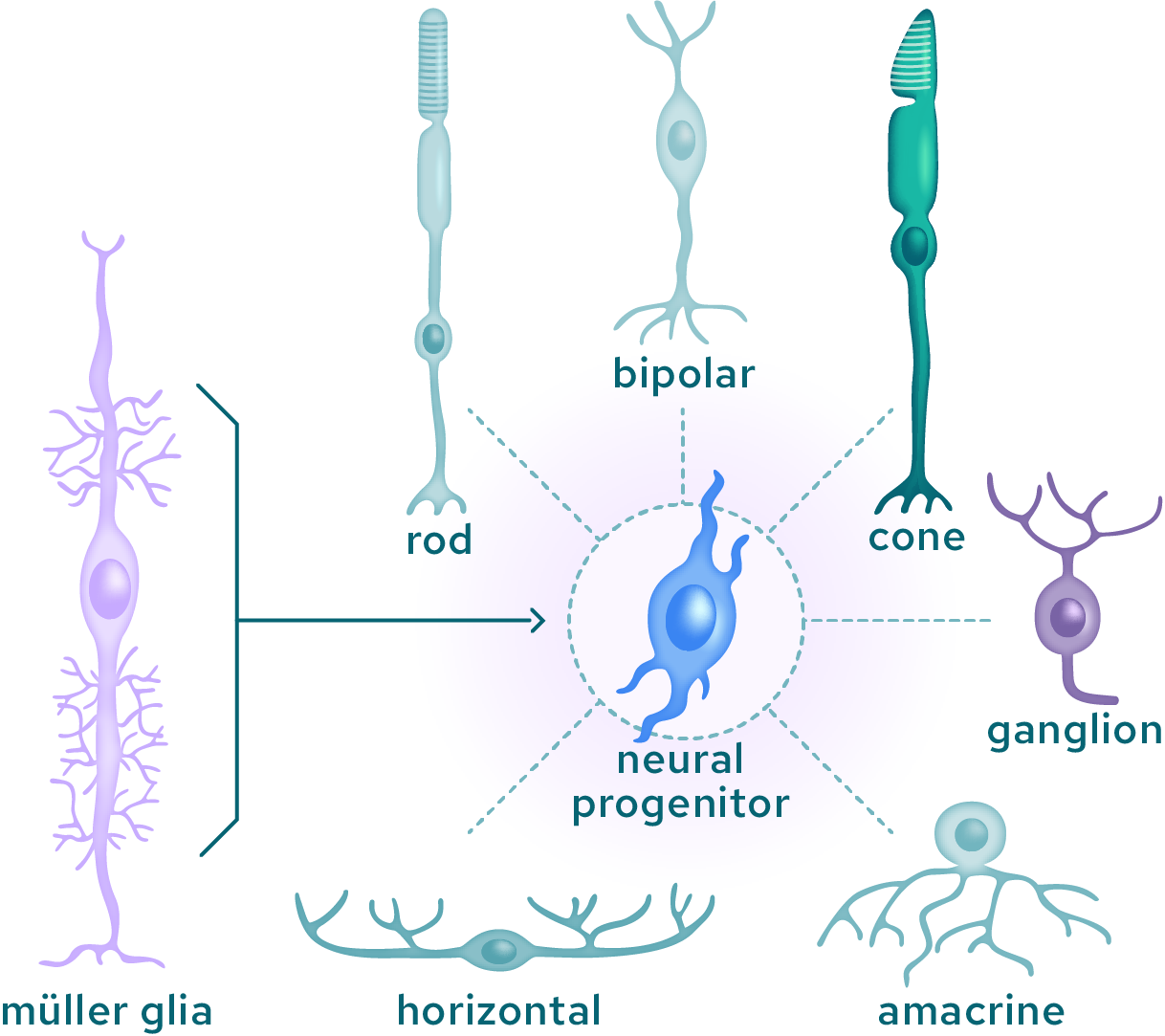
Our ex vivo approach begins with pluripotent stem cells, which have the potential to differentiate into any cell type and directs their differentiation into any of several specific ocular cell lineages. These differentiated cells are administered to the eye, where they integrate with existing structures to replace damaged or lost tissues.
Creation of pluripotent stem cells
Each one of us grew from a single cell with the capacity to generate every cell type present in our bodies. This inherent property, known as pluripotency, enables stem cells to develop a specific cellular identity in response to the activation of various regulatory genes.
Building on Nobel Prize–winning work, it is now possible for this differentiation process to be reversed, changing already-specialized cells back to their pluripotent state. The resulting cells, known as induced pluripotent stem cells (iPSCs), can then be re-differentiated into different cell types. Through this process, it is possible to create the highly specialized cells necessary for vision from iPSCs.
Differentiation into specialized ocular cells
There are a number of approaches to generate specialized ocular cells from pluripotent cells.
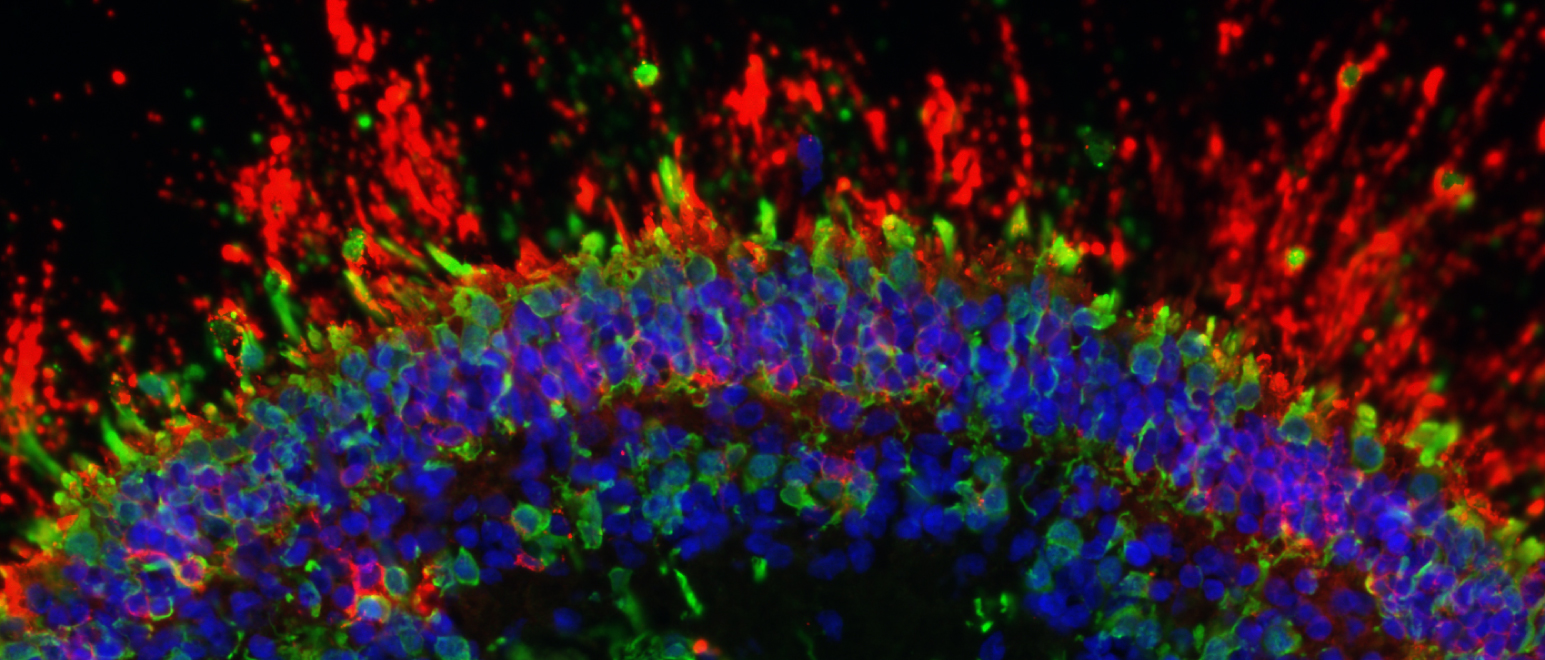
Neural cell types can be created by generating retinal organoids, which are three-dimensional structures containing primitive photoreceptors and other differentiated cells. Retinal organoids can be generated from iPSC when exposed to a particular combination of biochemical signals. Once matured, the photoreceptor precursor cells express light-sensitive pigments and transduce light into electrical signals. At the end of this maturation step, rod and cone photoreceptors are isolated and purified from the organoid to yield retinal photoreceptor progenitor cells. Additionally, retinal pigment epithelial cells can be created by exposing stem cells to specific chemical signals while they are attached to a specialized support matrix. This process creates islands of partially differentiated cells, which can then be selected and further expanded as fully differentiated cells.
Potential for genetic modification to improve function
In some applications there may be benefit from genetically modifying the pluripotent stem cells prior to differentiation, with the goal of improving performance of the cells, once they have been differentiated to the target cell type and transplanted. This may be achieved by deleting a gene (gene knock-out), adding a gene (gene knock-in), or through other modifications. We can perform any genetic modification at the stem cell stage, such that all subsequent differentiated cells will harbor this added genetically edited feature.
Transplantation into the eye
Advanced surgical techniques are available that enable the precise placement of new cells at the locations where they are needed.
In vivo
In nature, some animals such as fish and amphibians can regenerate specialized neuronal ocular cells after injury by reprogramming other structural cells in the eye. The latter first enter a progenitor like state that is further differentiated into selected cell types that replenish the lost structures. This approach has potential to regenerate a number of different neuronal ocular cell types.
Publications & Presentations
These are examples of research from Tenpoint’s founders and others that are representative of findings Tenpoint will build upon as it develops its pipeline.
- January 16, 2019
- Science Translational Medicine
- March 19, 2018
- Nature Biotechnology
- December 20, 2017
- Science Translational Medicine
- March 16, 2017
- The New England Journal of Medicine
- April 20, 2021
- Cell Reports
- October 04, 2019
- Nature Communucations
- January 09, 2019
- Development
- November 27, 2018
- eBioMedicine
- September 11, 2018
- Stem Cell Reports
- January 12, 2018
- Stem Cells
- November 23, 2022
- Science Advances
- October 19, 2021
- Cell Reports
- December 09, 2008
- PNAS

We have a shared vision
We believe that returning vision is among the most meaningful innovations that medicine can achieve. Our team is composed of leading experts who are dedicated to restoring sight for patients with degenerative eye disease.
Join Us